Special Offer: ONE-D Accelerated TMS Therapy for Just $1800!
Interested in ONE-D TMS Therapy?
Florida TMS Clinic offers ONE-D Accelerated TMS Therapy, which involves 20 iTBS treatment sessions in one day. We don't offer SAINT.
SAINT TMS Depression Treatment
Is 90% Too Good To Be True?
Khaled Bowarshi, M.D.
FLORIDA TMS CLINIC
Tampa, FL
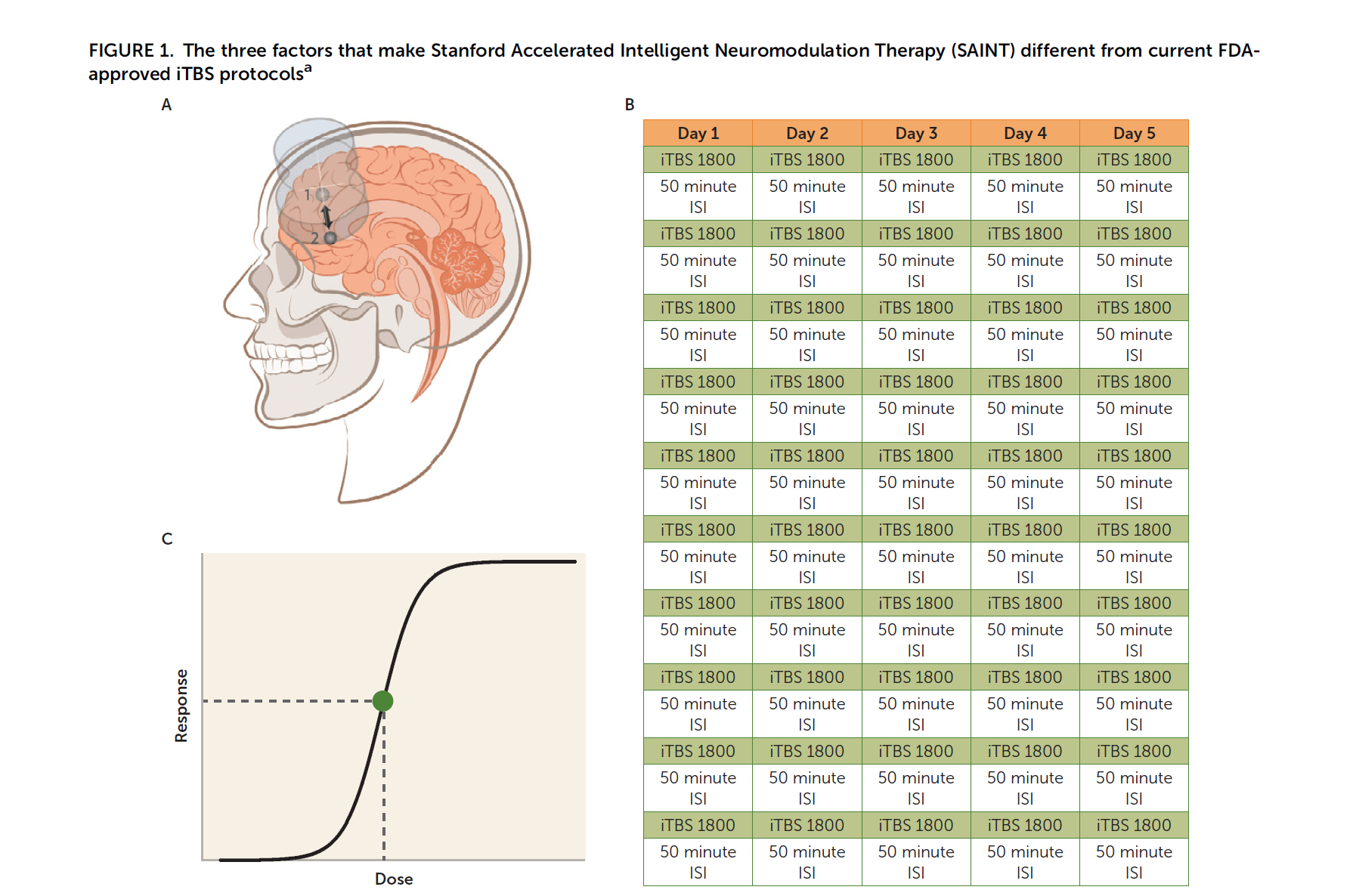
SAINT stands for Stanford Intelligent Accelerated Neuromodulation Therapy. SAINT depression treatment protocol uses a rapid form of Transcranial Magnetic Stimulation called Intermittent Theta Burst Stimulation, delivering 1800 TBS pulses in less than 10 minutes, every hour, 10 times a day, for 5 days. In total, delivering 50 treatment sessions equivalent to 90,000 pulses in 5 days. SAINT uses a unique brain imaging method to determine the magnetic stimulation's target area. SAINT reported a success rate of 90% at the end of SAINT treatment and 60% in a month from the treatment. The already FDA-cleared protocol of Repetitive Transcranial Magnetic Stimulation [rTMS] also delivers a total of 90,000 pulses but in 30 sessions, each session delivering 3000 pulses, done daily, 5 days a week for 6 weeks. Conventional rTMS has a success rate that doesn’t match up to the reported success rate of SAINT. Based on the reported results, SAINT was nothing short of a breakthrough in the field of neuromodulation. SAINT promised that a more dense TMS protocol could be superior. SAINT depression treatment got FDA-clearance in September 2022. Naturally, SAINT was an astonishing surprise to the old establishment in the field of TMS, creating a state of confusion for both patients and doctors. Here, I clarify this confusion. If you are here for small bites, no worries, I've got you covered. Just read the key takeaway points below and the one-paragraph summary at the end.
KEY TAKEAWAYS
- SAINT protocol is 1800 pulses of iTBS done every hour, 10 times a day, for 5 days.
- SAINT uses a resting functional connectivity MRI to specify the treatment target.
- SAINT reported an impressive success rate of 90% but dropped to 60% after a month.
- SAINT is now FDA-cleared. It is available at about 10 locations in the US, but it is costly.
- Accelerated TMS without fcMRI is not SAINT; a new one-day accelerated TMS protocol called ONE-D beats SAINT in practicality and cost. It doesn't require a complicated MRI and is more durable.
In this article, I will explain the history of accelerated TMS. The previously documented success of accelerated TMS. This will include accelerated left-sided high-frequency TMS, accelerated right-sided low-frequency TMS, and accelerated left-sided theta burst stimulation. I will touch on the concept of neuronavigation in the field of TMS. Finally, I will explain the SAINT protocol. I will summarize both SAINT, the open-label trial, and SNT, the randomized controlled trial. I will explain the flaws of some of the conclusions made in the headlines. I will then give my take on SAINT. I will divide this into three chapters:
Chapter 1: Accelerated TMS.
Chapter 2: Navigated TMS.
Chapter 3: Accelerated fcMRI NeuroNavigation TMS = SAINT.
Chapter 1: Accelerated TMS
Accelerated Transcranial Magnetic Stimulation | Terminology, History, and Importance.
Traditional daily TMS therapy has been used in clinical psychiatric practices since 2008 following its clearance by the FDA. Despite the alternative it provided, rTMS was challenged by the inconvenience of the duration of the treatments. A typical rTMS therapy session takes 37 minutes. The duration can be shortened to 18.5 minutes using the “Dash” protocol. The " Dash " protocol shortened the intra-train interval [ITI] from 26 to 11 seconds. Despite the time efficiency “Dash” protocol provided, accounting for the entire course being 30-36 sessions, it is still a lot of time on the treatment chair. It became obvious that we need more efficient and practical treatment. Hence comes accelerated TMS. Trying to accelerate TMS treatment is as old as TMS itself. Let me be clear here; accelerated TMS was proposed a decade before Stanford entertained the idea of accelerated TMS. It was even before Theta Burst Stimulation clearance by the FDA (read about accelerated iTBS below). In the medical literature, the term accelerated TMS is used to describe any TMS therapy protocol applying more than one TMS session a day. Accelerated TMS therapy is exciting because of many reasons. The main reasons are:
- Accelerated TMS can be more effective than traditional daily TMS.
- Accelerated TMS can be more practical for some patients who can’t commit to 6 weeks of treatments and prefer just a few days.
- Accelerated TMS can improve access to patients who need it the most. Particularly those hospitalized with severe depression.
Evidence Supporting Accelerated TMS
We are going to discuss three main methods of delivering accelerated TMS. SAINT, as a category of accelerated TMS, falls under the third category.
- Left-Sided High-Frequency Accelerated TMS
- Right-Sided Low-Frequency Accelerated TMS
- Accelerated Intermittent Theta Burst Stimulation
1. Left-Sided High-Frequency Accelerated TMS
Clinical research supports the efficacy of accelerated TMS therapy for depression treatment. The vast majority of the work is done outside of Stanford and is completely independent of it. Stanford's work was more like the icing on the cake. Let’s review some of the published evidence.
In 2010, an open-label accelerated TMS (aTMS) trial was done at Emory. It consisted of 15 rTMS sessions administered over 2 days and treated 14 depressed patients. Accelerated TMS demonstrated an excellent safety profile with efficacy comparable to that achieved in daily rTMS in other trials. Here is the PubMed link to the trial.
In 2014, a naturalistic trial result from Canada came out, 27 patients with moderate to severe chronic and treatment-resistant MDD were treated with twice-daily HF-rTMS (10 Hz) applied over the left dorsolateral prefrontal cortex for 2 consecutive weeks (60,000 pulses). Ten (37.0%) patients met the criteria for clinical remission, and 15 (55.6%) were classified as responders. This meant that an accelerated protocol involving twice-daily sessions of HF-rTMS over the left DLPFC for 2 weeks was effective in treatment-resistant MDD and had excellent acceptability. Here is the PubMed link.
In 2017, a very well-respected Canadian research group, including Dr. Daniel M. Blumberger and Dr. Jonathan Downar. reported a retrospective chart review of 130 patients with MDD who went through either twice-daily rTMS or once-daily rTMS for 20-30 treatment sessions. They concluded that twice-daily rTMS appears feasible, tolerable, and capable of achieving comparable results to once-daily rTMS while also reducing course length approximately twofold. Therapeutic gains tracked the cumulative number of sessions, not pulses. Here is the link to the abstract.
Another group in Canada compared twice-a-day rTMS (17 patients) to once-a-day rTMS (19 patients). The majority of patients in both groups responded to treatment, and there was a trend toward a greater response rate in the twice-daily group (82.4%) compared to the once-daily group (52.6%). Patients in the twice-daily group experienced an improvement in symptoms faster than in the once-daily group due to the accelerated therapy period. The conclusion was made that the administration of two rTMS treatment sessions per day is tolerable for patients and does not seem to be inferior in efficacy to a once-daily protocol. Twice-daily administration has the benefit of producing symptom improvement over a shorter time span and requires fewer visits to the clinic. This was published in the Neuropsychiatric Disease and Treatment in 2018. Here is the PubMed link.
Dr. Fitzgeral (a star in the field of TMS in Australia) and his group designed a study comparing accelerated TMS to traditional TMS. They devised an approach consisting of 3 weeks of decreasing treatment intensity. In week 1, patients were provided 3 treatments per day over 3 days. In week 2, 3 treatments over 2 days were provided, and in week 3, 3 treatments on a single day were provided. Each treatment day involved a total of 250 10 Hz trains, so the total dose of TMS provided across 6 days was equal to that provided in 20 days of treatment at 75 trains per day. 115 outpatients with MDD received either accelerated rTMS (n = 58) or standard rTMS (n = 57) following randomization. There was no significant difference in response rates or remission rates between the groups in any of the analyses. This was published in 2018. PubMed link.
Another Canadian group did a retrospective data analysis of 73 patients with unipolar and bipolar depression who went through accelerated rTMS of 30 treatment sessions in 3 weeks (2 sessions a day). The overall outcome was positive but more notable for those over the age of 60. They concluded that the accelerated rTMS protocol is a safe and effective treatment for unipolar and bipolar depressed subjects, including older adults. This was published in the American Journal of geriatric psychiatry in 2019. PubMed link.
In 2020, my colleagues at WVU explored accelerated TMS on 6 post-stroke depression patients using High-frequency (20-Hz) rTMS at 110% resting motor threshold (RMT) was applied to the left dorsolateral prefrontal cortex (DLPFC) during five sessions per day over four consecutive days for a total of 20 sessions. All 6 patients remitted and maintained remission at 3-months follow-up. Here is a link to the paper.
2. Right-Sided Low-Frequency Accelerated TMS
It is already established that right-sided low-frequency TMS can also help with depression. Low-frequency TMS (1 Hz) is thought to carry a lower seizure risk than high-frequency TMS. If we were to give multiple TMS sessions in a day and we were concerned about safety, it would make a lot of sense to consider low-frequency TMS.
Some primary work was done in 2016. An open-label accelerated rTMS pilot was done with 7 treatment-resistant patients (4 unipolar, 3 BP). Accelerated rTMS was given over the right dorsolateral prefrontal cortex at 120% of the resting motor threshold at 1 Hz, and 900 pulses were delivered per session. A single rTMS treatment was administered on the first day to test for tolerability, followed by 5 rTMS sessions a day for 2 days, then 7 days of daily rTMS sessions. The total course consisted of 16,200 pulses across 18 sessions given over 10 consecutive weekdays. Though the efficacy was mediocre, the authors found accelerated low-frequency right-sided rTMS was a safe and possibly efficacious treatment for treatment-resistant depression. Here is the link.
Recently in 2021, Dr. Downar and his group in Canada did a feasibility study of a 5-day accelerated 1 Hz rTMS protocol. They conducted a prospective, single-arm, open-label feasibility study. Thirty (30) participants received a one-week (5 days) accelerated (8 sessions per day, 40 sessions total) course of 1 Hz rTMS (600 pulses per session, 50-minute intersession interval) over the right dorsolateral prefrontal cortex (R-DLPFC) using a figure-of-eight coil at 120% of the resting motor threshold (rMT). Response and remission rates 1 week after treatment were 33.3% and 13.3%, respectively, and increased to 43.3% and 30.0% at follow-up 4 weeks after treatment. This meant that 1 Hz rTMS administered 8 times daily for 5 days is safe and well-tolerated. Link to the study.
Then, in the same year 2021, they published their proof of concept doing right-sided low-frequency accelerated TMS targeting the right DLPFC using F4 (no neuronavigation). Forty-eight (48) MDD participants received an initial accelerated rTMS course (arTMS) of 6 sessions/day over 5 days (30 total), followed by a tapering course of daily sessions (up to 25) to decrease the odds of relapse. Response and remission rates were 35.4% and 27.1%. This trial was of unique importance because it used the most cost-effective method of delivering accelerated TMS. Click here to read the paper.
3. Accelerated Intermittent Theta Burst Stimulation
As we saw above, there is adequate evidence supporting accelerated TMS using the older rTMS frequency of 10 Hz, which is the standard high-frequency protocol that was FDA-cleared in 2008. Fast forward 10 years to 2018, a breakthrough in TMS called iTBS got FDA-cleared. Intermittent Theta-Burst Stimulation (iTBS) is the “version 2.0” of rTMS. Just like rTMS, iTBS is a non-invasive brain stimulation treatment that is FDA-cleared for treatment-resistant depression (TRD). It uses a different magnetic pulse (in triplets) at a unique frequency, delivering the treatment in just 3 minutes and 12 seconds. iTBS was proven to be as effective as rTMS, with the obvious time saving of over 5-fold. This opened the door wide open to an even more aggressive accelerated treatment protocol.
A significant amount of research done exploring accelerated TBS started in Europe, particularly in Belgium. Based on a 2014 study, it was concluded that Accelerated TBS treatment in depressed, suicidal patients is safe and well-tolerated and may potentially decrease suicidal ideations. Here is the link. A couple of years later, in 2016, they published in the Journal of Affective Disorders in 2016 that 20 sessions of iTBS given 5 sessions a day for only four days of accelerated iTBS treatment applied to the left DLPFC in TRD may lead to meaningful clinical responses within two weeks after stimulation. Here is the PubMed link. Then in 2018, the group did a retrospective comparison between accelerated high-frequency rTMS (arTMS) with accelerated intermittent theta burst stimulation (aiTBS) in the refractory depressed state. The clinical efficacy was not significantly different between both protocols. This substantiates the potential of the accelerated stimulation to shorten the treatment duration from the depressed state to the response state. Any time gained from the depressed state to the recovered state is in the patient's interest. Here is a link to the publication.
In 2019, Erine Bröcker and colleagues reported on accelerated theta-burst repetitive transcranial magnetic stimulation for depression in South Africa. In their report, 9 patients (7 with MDD and 2 bipolar depression) went through 20 iTBS sessions in 8 days spread over 2 weeks. 1782 pulses per session at 80% MT using an F8 coil without neuronavigation. Five of the nine participants were responders. This study was received in 2018 and published in 2019. See the link.
In 2020, Dr. Muir Owens and his group at Brooklyn Minds published an analysis of collected data on accelerated and non-accelerated dTMS protocols. One hundred eight patients received dTMS treatment for depression. Stimulation with H1 coil was administered to the left prefrontal cortex (LPFC) at 80% MT intermittent theta burst (iTBS) 600 -1800 pulses with an intertreatment interval of fifty minutes. Patients received a range of treatments between 3 and 92 sessions (m = 42.94). Of those 107 patients, 21 received an accelerated protocol which consisted of, a range of 3 to 10, treatments in a day. The other 87 patients received a non-accelerated protocol. Results indicate that both accelerated and non-accelerated protocols of dTMS reduced depression symptoms in MDD patients. See the link to Brain Stimulation Journal.
In 2021 my group and I at FLORIDA TMS CLINIC reported retrospective data analysis of non-MRI-guided accelerated intermittent theta burst stimulation. Six patients with depression, five unipolar and one bipolar II, all had severe treatment resistant depression (> 4 antidepressant history), two with a previous history of receiving TMS therapy, received accelerated iTBS. Stimulation with F-8 coil was administered to the left dorsolateral prefrontal cortex (DL-PFC) determined using StimGuide, a non-MRI-navigation system. Stimulation was at 90% MT intermittent theta burst (iTBS) for 1800 pulses with an intertreatment interval of fifty minutes. Patients received ten sessions every day for five consecutive days for a total of fifty sessions. Five out of the six patients responded to accelerated iTBS. Results indicate that non-MRI-guided accelerated intermittent theta burst stimulation reduced depression symptoms in depressed patients with treatment resistance. See the link to Brain Stimulation Journal.
In 2021, The Australian group with Dr. Fitzgerald in Australia published a RCT comparing rTMS with accelerated iTBS unilateral and bilateral. The overall treatment response rate was 43.7 %, and the remission rate was 28.2 %. There were no significant differences for response or remission across the three groups. Here is the PubMed link.
Chapter 2: Navigated TMS
How about the evidence for neuronavigation targeting? Is Neuronavigation targeting superior to scalp measures targeting? The multi-million dollar question!
I will divide this topic into three sections:
- The case for neuronavigation.
- The case against neuronavigation
- fcMRI neuronavigation targeting
1. The case for Neuronavigation
MRI navigation might be better... At least on paper!
Dr. Fitzgerald and colleagues investigate whether repetitive transcranial magnetic stimulation (rTMS) targeted to a specific site in the dorsolateral prefrontal cortex (DLPFC), with a neuro-navigational method based on structural MRI, would be more effective than rTMS applied using the standard localization technique. Fifty-one patients with treatment-resistant depression were randomized to receive a 3-week course (with a potential 1-week extension) of high-frequency (10 Hz) left-sided rTMS. Thirty trains (5 s duration) were applied daily 5 days per week at 100% of the resting motor threshold. Treatment was targeted with either the standard 5 cm technique (n=27) or using a neuro-navigational approach (n=24). This involved localizing the scalp location that corresponds to a specific site at the junction of Brodmann areas 46 and 9 in the DLPFC based on each individual subject's MRI scan. There was an overall significant reduction in the Montgomery-Asberg Depression Rating Scale scores over the course of the trial, and a better outcome in the targeted group compared with the standard localization group at 4 weeks (p=0.02). Significant differences were also found on secondary outcome variables. The use of neuro-navigational methods to target a specific DLPFC site appears to enhance response to rTMS treatment in depression. Further research is required to confirm this in larger samples or to establish whether an alternate method based on surface anatomy, including measurement from the motor cortex, can be substituted for the standard 5 cm method. See link to paper.
2. The case against Neuronavigation
Navigation is not better… At least not clinically relevant.
The Canadian group behind THREE-D answered the question by quantifying the discrepancy in scalp site between BeamF3 versus MRI-guided neuronavigation for left DLPFC. Out of 100 subjects, they found that BeamF3-to-MRI-guided discrepancies were <0.65 cm in 50% of subjects, <0.99 cm in 75% of subjects, and <1.36 cm in 95% of subjects. This meant that the BeamF3 heuristic may provide a reasonable approximation to MRI-guided neuronavigation for locating left DLPFC in a majority of subjects. See this link. The group used these findings in their final recommendations from the THREE-D trial. Though three THREE-D trial used MRI navigation, they didn’t justify the added cost. Hence, the FDA approved iTBS based on the THREE-D trial without navigation. See this link.
A study in Taiwan published in 2019, showed that the MRI-guided method of coil targeting is not better than the standard method. This group did prolonged intermittent theta-burst stimulation (piTBS) with triple doses of the standard protocol. The primary objective was to investigate the antidepressant efficacy of piTBS monotherapy. Efficacy between the standard 5-cm method and magnetic resonance imaging (MRI)-guided coil positioning to the left dorsolateral prefrontal cortex method was also compared. This was a double-blind, randomized, sham-controlled trial, 105 patients with recurrent depression who exhibited no responses to at least one adequate antidepressant treatment for the prevailing episode were assigned randomly to one of three groups: piTBS monotherapy (n = 35), repetitive transcranial magnetic stimulation monotherapy (n = 35), or sham stimulation (n = 35). The acute treatment period was 2 weeks. Half of the patients were randomized to MRI navigation in each group. The piTBS group exhibited significantly greater decreases in depression scores than the sham group at week 2 (-40.0% vs. -13.9%). The MRI navigation method (-32.4%) did not yield better antidepressant effects than the standard method (-40.6%). Read the paper here.
A more recent publication from Germany in 2021 also showed no superiority of MRI navigated TMS. This randomized controlled trial investigated whether a four-week course of neuronavigated intermittent theta burst stimulation (iTBS) of the left dorsolateral prefrontal cortex is superior to the non-neuronavigated F3-EEG method of positioning. 37 inpatients with at least moderate depressive episode were randomized to receive either neuronavigated or 10-20-EEG-system based F3 guided iTBS. Both groups received twenty daily sessions of iTBS while continuing to receive standard-of-care treatment by their ward physicians. The number of remitters was exactly the same for both groups. The investigators noticed a high antidepressive effect of add-on iTBS treatment to standard inpatient treatment but failed to demonstrate a clinical superiority of neuronavigated localization. The non-navigated, F3-guided iTBS treatment used as a control group may be sophisticated enough to dilute potential added benefits, and the difference between the localization approaches is either negligible or too small to justify the additional efforts of navigation. See this link.
3. Functional Connectivity MRI Targeting
How about functional connectivity MRI targeting? That must be better. Or is it?
Parallel to the work that was done exploring accelerated TMS and accelerated TBS. Others were exploring optimizing the treatment location. A group from Ghent University, Belgium, reported that the anti-correlation between the sgACC and parts of the left prefrontal cortex could be indicative of a beneficial outcome. This group was also investigating accelerated HF-rTMS treatment designs to have the potential to acutely adjust deregulated sgACC neuronal networks in TRD patients. This dates back to 2014. Here is the link.
Dr. Michael D. Fox and his group at MGH reported that the antidepressant efficacy of different left DLPFC TMS sites is related to the anticorrelation of each site with the subgenual cingulate, potentially lending insight into the antidepressant mechanism of TMS and suggesting a role for intrinsically anticorrelated networks in depression. These results can be translated into a connectivity-based targeting strategy for focal brain stimulation that might be used to optimize clinical response. Here is the link.
Probably the best summary of the argument about the location of treatment was done by Dr. Fitzgerald. In his paper, he discusses all the data available up to 2021. fMRI-connectivity-based approaches to targeting specific circuits in the DLPFC are intellectually attractive but it may not be possible to demonstrate differential effectiveness of these over the methods most commonly been used in clinical practice. He concludes There is emerging literature helping to improve our understanding of the optimal methods for targeting rTMS treatment for depression. However, we lack substantive prospective clinical trials demonstrating improved clinical outcomes with these techniques. See the link here.
The most recent publication on the topic of neuroimaging targeted TMS and TBS was published in March of 2023. In this paper, analysis of about 300 patients from the THREE-D trial indicated that in a large representative sample of patients with major depressive disorder, individual differences in sgACC-StimFC explained only ∼3% of the variance in outcomes, which may limit the utility of existing sgACC-based targeting protocols. This again shows that functional neuroimaging targeting might not be as high yield as it sounds.
Chapter 3: SAINT
Functional Connectivity MRI Targeted High Dose Accelerated Theta Burst Stimulation
Now that we know what accelerated TMS and accelerated iTBS are. We already learned about the controversy surrounding the superiority of fcMRI navigation. Let’s couple the two together. aiTBS + fcMRI = SAINT.
What is SAINT Depression Treatment Protocol?
Today, the terminology accelerated TMS and accelerated TBS is commonly used to describe the TMS therapy protocol in which the patient gets 10 TMS therapy sessions a day for 5 days for a total of 50 TMS therapy sessions. This accelerated TMS therapy protocol shortens the duration of treatment from 6 weeks to just 5 days.
In the beginning, Dr. Nolan Williams examined the effects of multiple iTBS treatments per day in a small sample of patients who did not previously respond to a full course of rTMS and an acute course of ECT. The pilot study was published in 2018 in which accelerated TMS was used to treat 6 patients with highly refractory MDD patients using intermittent theta-burst stimulation (iTBS), 1800 pulses/session, 10 sessions per day over 5 days, and reported remission in 5 out of the 6 patients at day 5. All patients were back into depression 4 weeks after the treatment. See the link. This raised the question about the “fast on / fast off” phenomenon of accelerated TMS. Read more about it in this link. That pilot study paved the road for SAINT.
SAINT Depression Treatment is an accelerated TMS protocol that came from Stanford University and was published in the prestigious peer-reviewed American Journal of Psychiatry in April 2020. See the PubMed link. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression (SAINT-TRD) proved the efficacy and safety of accelerated TBS protocol for depression. In SAINT clinical trial, 21 participants with TRD received 50 iTBS sessions as 10 daily sessions over 5 consecutive days. Amazingly, 19 of 21 participants (90.48%) met the criteria for remission immediately after SAINT. In the intent-to-treat analysis, 19 of 22 participants (86.4%) met remission criteria. Neuropsychological testing demonstrated no negative cognitive side effects. This supported that accelerated iTBS was well tolerated and safe. Efficacy was surprisingly high.
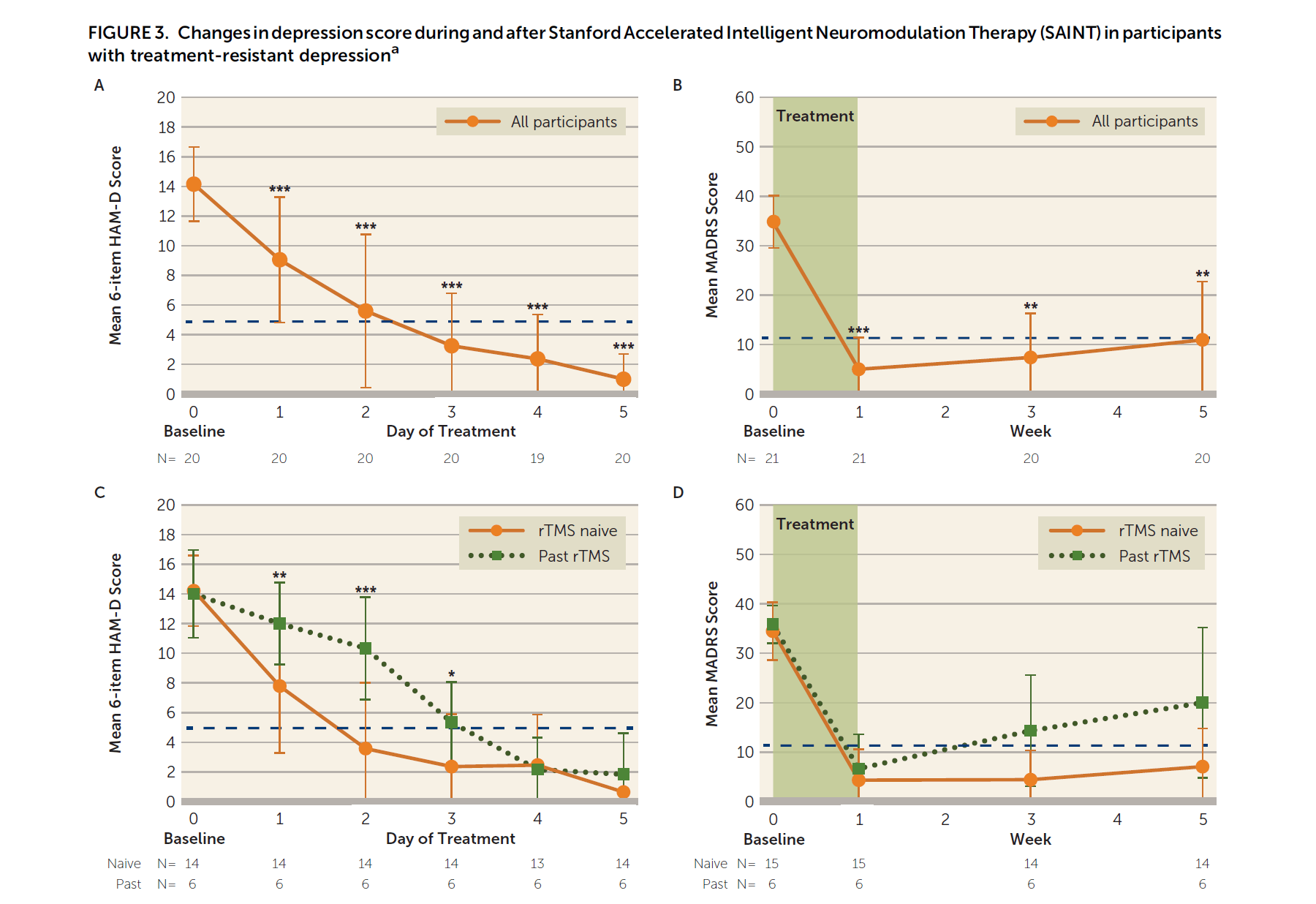
Is SAINT too good to be true?
Let’s talk numbers.
SAINT response rate on day 5 was 85-90% (depending on the rating scale).
SAINT response rate at week 5 was 60-70% (depending on the rating scale).
If we agree that the meaningful outcome should not be at day 5 but rather at a point of time similar to the 4-6 weeks of traditional TMS treatment. A 60-70% response/remission rate should have been reported to avoid the “too good to be true” dilemma. But even with a 60-70% success rate, this is nothing short of a breakthrough!
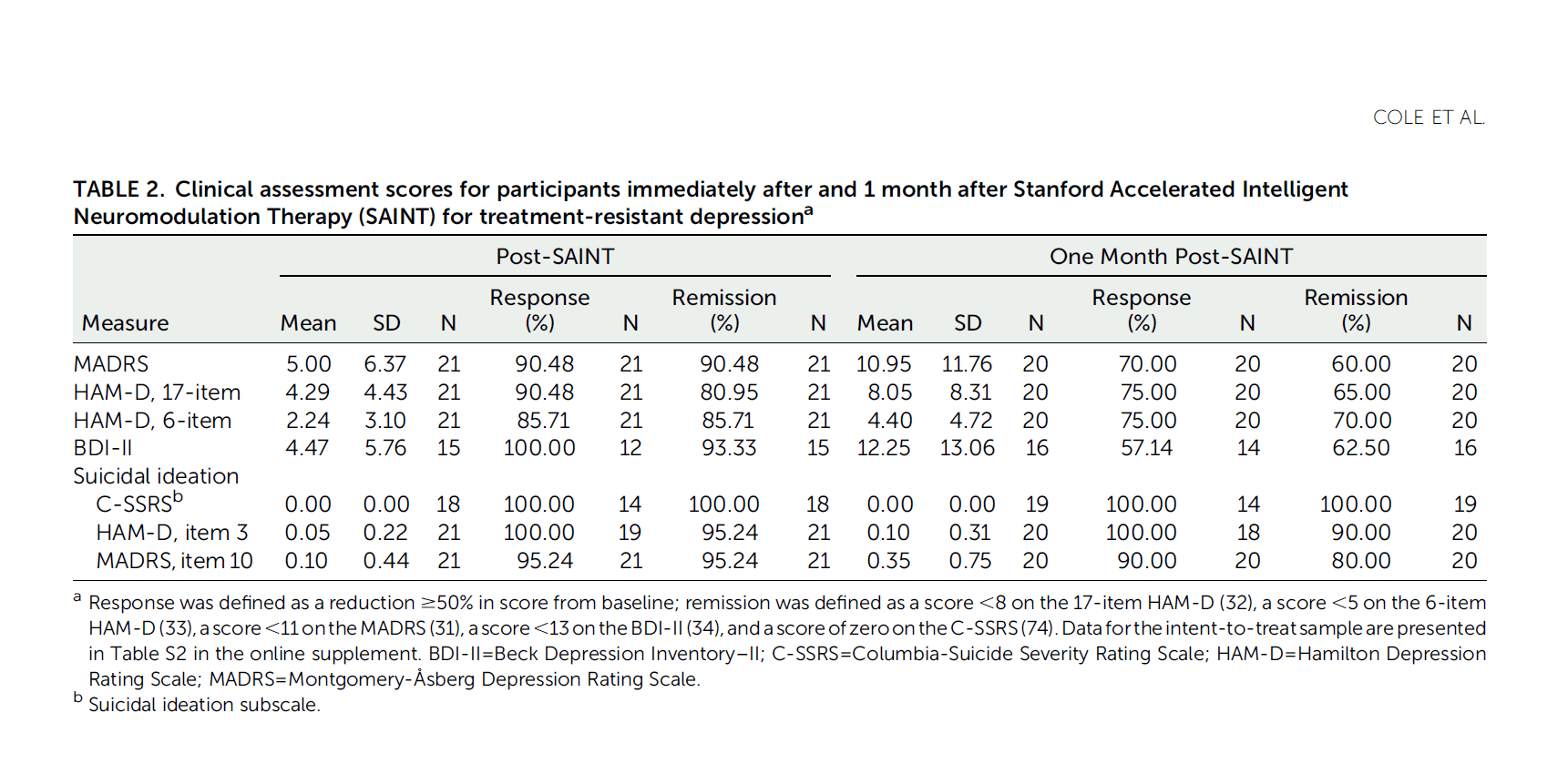
What is SNT Depression Treatment Protocol?
SNT is Stanford Neuromodulation Therapy (previously referred to as Stanford Accelerated Intelligent Neuromodulation Therapy or SAINT). SAINT and SNT are exactly the same. It is more of a branding exercise. It seems like the main goal was to avoid the word “accelerated” because, as you read above, Stanford didn’t invent the idea of accelerated TMS therapy. Rather, optimized it. That being said, SAINT was more catchy than SNT. A quick Google trend check would show that patients are primarily looking up SAINT depression treatment, Stanford TMS protocol, or just accelerated TMS, ignoring the terminology SNT and ignoring the terminology Neuromodulation.
Besides branding, SNT was the name of the randomized controlled trial that applied the SAINT protocol. SNT results were revealed in late 2021 in the American Journal of Psychiatry. In the SNT trial, 32 participants with treatment-resistant depression were enrolled, and 29 participants who continued to meet inclusion criteria received either active (N=14) or sham (N=15) SNT. Just like SAINT (open-label trail), SNT (randomized controlled trial) coupled iTBS with individualized circuit-based neuronavigation and an accelerated treatment schedule. SNT found a 52.5% drop in depression scores with active SNT compared with an 11.1% drop with sham. From the research design aspect, this proves the effectiveness of accelerated TMS over sham (placebo device). This is a huge step forward for the FDA clearance. Here is a link to the paper.
In SNT (randomized controlled trial), the response rate immediately after the treatment (day 5) rate was 85.7% (12 out of 14) which dropped to 69.2% (9 out of 13) one month after (week 5). The remission rate immediately after the treatment (day 5) rate was 78.6% (11 out of 14) which dropped to 46.2% (6 out of 13) one month after (week 5).
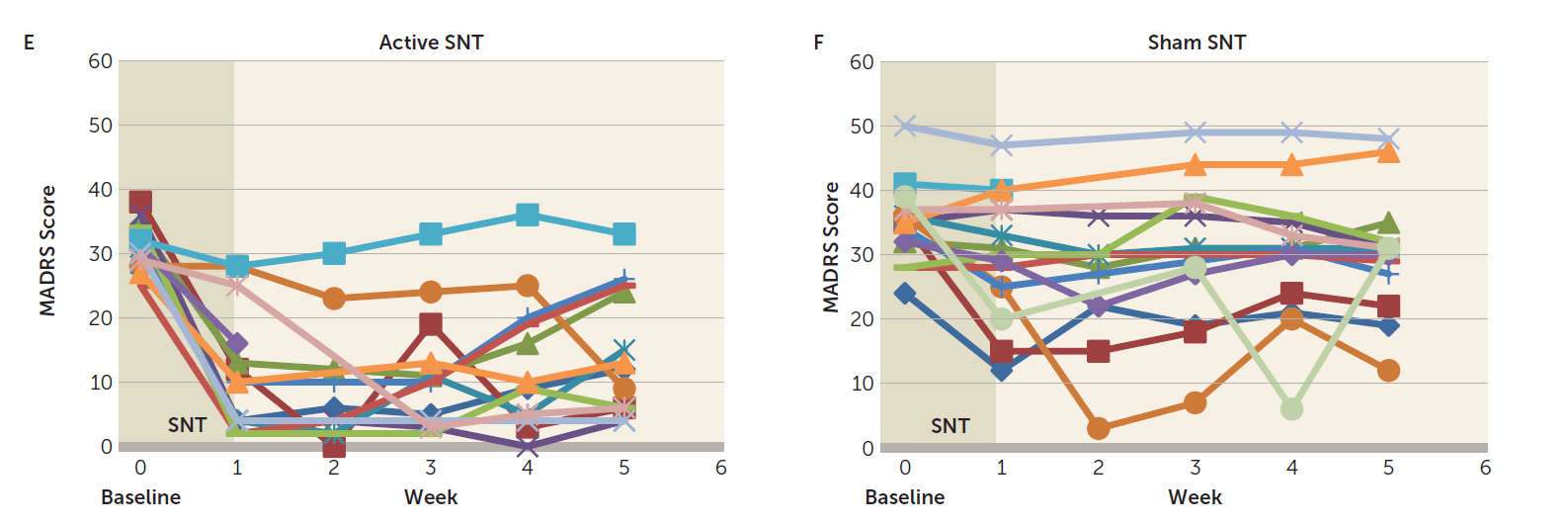
Key differences in results reporting in SAINT vs. SNT
Unlike SAINT, participants with prior exposure to rTMS were excluded from enrolling in SNT. It seems that the authors picked on the pattern noted in SAINT with a delayed response and higher relapse in those with previous TMS treatment. They decided to exclude patients with a previous history of TMS in the RCT, increasing their chances of separation. Maybe they had to exclude patients previously treated with TMS for better blinding, but it is still worth noting.
SNT reported success as remission at any week during the next five weeks after SNT treatment. It is unusual to report remission and response for just one out of five follow-up points. The remission rate at week 5 was 46.2%, and the response rate at week 5 was 69.2%. Don’t take me wrong, these numbers are excellent. But less earthshattering than the 90% number picked up by the media and press releases.
What did SNT prove?
SNT as a blinded randomized controlled trial proved that accelerated TMS is effective. This eliminates any doubt about SAINT efficacy being a placebo effect. When it comes to the proof for fcMRI targeting, the picture becomes less clear. That’s because SNT/SAINT already used three components with a high probability of increasing the efficacy of treatment. SAINT added a fourth ingredient which is the fcMRI-targeting that we are not very sure about.
The three proven ingredients for SAINT/SNT success.
1. Dose per session:
The dose of iTBS is X3 folds the FDA-cleared iTBS dose. That’s 1800 pulses in SNT vs. 600 pulses in THREE-D.
2. Density:
The density of the treatment course was over X20 folds over the FDA-cleared course. The density of TMS is the frequency of treatments (frequency of treatments is the number of treatment sessions per day, not to be confused with pulse frequency which is number of TMS pulses per second and measured in Hz). SNT did 50 minutes between sessions instead of 24 hours between sessions in THREE-D.
3. Number of sessions:
SNT did a total of 50 sessions instead of 20-30 in THREE-D.
We already know that more pulse can lead to a better response. More treatment sessions can lead to a better response, and a more dense treatment course is better than a spread-out treatment course. Putting these three factors together. It makes a lot of sense that SNT was effective.
The one unproven ingredient for SAINT/SNT success:
fcMRI
SNT doesn’t prove the superiority of fcMRI-guided targeting. That is because SNT missed a golden opportunity to add a third arm doing accelerated with conventional targeting. Perhaps SNT investigators were aware that SNT and sham would separate very easily, and 60 participants would be more than enough to power the study for this separation. In reality, they didn’t even need 60 participants and stopped at 29 because of the obvious separation. On the other hand, fcMRI-SNT vs. non-MRI-SNT will need a couple of hundreds of study participants to stand any chance of separation. Unfortunately, this missed opportunity is going to cost us a lot of money on unnecessary imaging or a delay of a few years until the question of neuroimaging is answered once and for all.
On the topic of neuroimaging targeting, we need to understand that SNT used functional neuroimaging in addition to the structural MRI taking during such an fcMRI process, it wasn't just a structural neuroimaging. If you want to learn more, I wrote about the different types of navigated TMS. Resting functional neuroimaging requires sophisticated technology, a technician, a standardized protocol, and an experienced neuroradiologist. Many of these are readily available for mass production. Please remember that neither SAINT nor SNT was a multisite clinical trial. Replicating the same imaging standards in a multicenter clinical trial and then in real life to produce the same quality is going to be challenging, to say the least.
Cortical Depth Adjusted Intensity... Necessary or Gimmick?
SAINT and SNT added a unique intensity target of 90% MT depth adjusted up to 120%. So what was that about?
As you probably know from using your cell phone. The closer you are to the 4G or 5G tower, the stronger the signal is. The same principle applies to TMS. The closer the magnetic coil to the brain cortex, the stronger the stimulation. Based on that, SAINT research designers went fancy and decided to stimulate at 90% MT adjusted (up to 120%) based on the distance between the scalp and brain cortex. The larger the distance (depth), the more stimulation intensity but not to exceed 120% of resting Motor Threshold [rMT]. While it sounds smart, I think it ignores other aspects of simple physics. There are an unlimited number of other variants that need to be taken into consideration beyond depth. We can’t just pick one and ignore the others. Here are some examples:
- Bone density
- Skin type
- Hair type
- Body composition (subcutaneous fat)
- Age
- Gender
- Race
As you can see, the list can go on and on. For the calculation of intelligent neuromodulation to be meaningful, we either have to take all of it into consideration. Or we can just standardize the treatment to 120% of resting MT, which was repeatedly deemed safe.
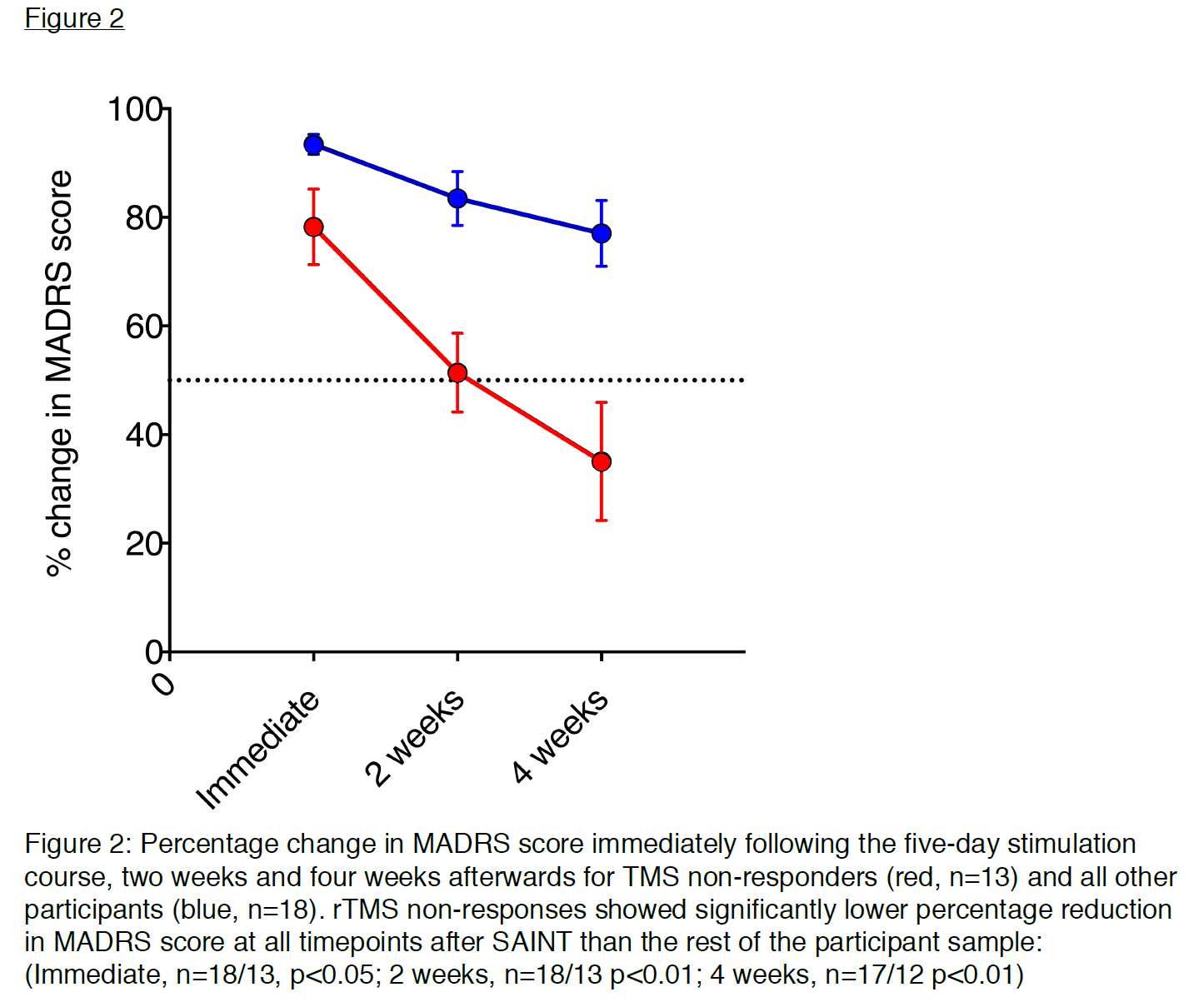
SAINT Durability: The "fast on / fast off" phenomenon of SAINT & SNT
Let’s make the observation that in both SAINT and SNT, about one-third of patients who responded/remitted with SAINT protocol lost the response/remission after a month. This was addressed in the section above when we discussed the pilot trial in which all responders relapsed. It is worth noticing that the less treatment-resistant the participants are, the better durability at the one-month follow-up. This can be observed in participants' selection from SAINT to SNT. SNT participants were less treatment-resistant compared to SAINT. In the pre-print copy of SAINT, which had 31 participants, 13 of them had failed a previous rTMS. These 13 failed-rTMS-participants responded less than "TMS-naïve" participants both immediately and 4 weeks after SAINT. The separation was much more obvious at week number 4. In other words, higher treatment resistance (previous failure to rTMS) is a negative predictive factor for response and a much more negative predictive factor for durability at week number 4 after SAINT. Interestingly, only 6 rTMS-failed-participants made the cut to be included in the published SAINT. Moreover, no rTMS-failed patients were recruited for the SNT trial. All of which helped massage the durability number in the final publication. I am sure there are valid reasons why these changes were made, but nevertheless, we need the durability question to be answered.
Summary:
In summary, SAINT/SNT might be the biggest breakthrough in neuromodulation to date. That being said, The 90% success rate thrown around in the media is too good to be true. A better report should be ~70% response rate and ~50% remission rate after a month. We are not yet sure we need individualized neuroimaging to get the efficacy of aiTBS. We are not completely confident about the long-term durability of SAINT, especially for those with previous failed TMS. And lastly, we can’t really give a blanket statement that SAINT is superior to conventional rTMS, but for sure, we can recognize that SAINT can be more practical solution for some patients.
SAINT is FDA-cleared now and is available at a few treatment centers certified by Magnus Medical. Please visit Magnus's website for a complete list of SAINT centers. Of note, the cost at some of these centers is $28,000, making access to SAINT more theoretical than practical.
The availability of the ONE-D accelerated TMS protocol, which doesn't require a complicated MRI and is more practical and durable, raises questions about SAINT adoption.
Conflict of Interest Disclosure:
FLORIDA TMS CLINIC and Dr. Khaled Bowarshi have no financial affiliation with Stanford or Magnus Medical. Florida TMS Clinic offers ONE-D Accelerated TMS Therapy, which involves 20 iTBS treatment sessions in one day. We don't offer SAINT.