TMS Devices Review
Updated Sep 2025
Before we dive into the different
TMS Therapy machines, I have to make a few disclaimers:
FloridaTMSClinic has no financial affiliation with any of the TMS manufacturers. I (the author, Dr. Khaled Bowarshi) don’t own any shares or stocks in any TMS company. These are my personal opinions based on my experience with these devices in my practice, testing at exhibits and live demos, and visiting some colleagues to check out devices.
This list is made in an order that I can explain the technology. There are a few criteria I use to evaluate a TMS device and the parent manufacturer:
- Ease of use.
- Quality of the product, technical support.
- Research and innovation.
- Transparency. Manufacturers who make false claims of superiority will lose points.
- Affordability and business model. Manufacturers who adopt the “silent partner” approach by the pay-per-use model lose points.
- History and experience in the field.
I added a rough estimate of the cost. Please note that pricing could change based on the market. Some manufacturers use the pay-per-use model. For those, I considered the cost of the machine upfront + treating 50 patients in year one before giving an overall cost rating.
NeuroStar
Neuronetics makes NeuroStar TMS. Neuronetics was created in 2003 and funded the pilot studies that came up with the conventional 10 Hz TMS therapy protocol as we know it today. Neuronetics sponsored the main pivotal trial published in Dec 2007 and got the first FDA clearance for TMS therapy in 2008. This makes NeuroStar the first FDA-cleared TMS in the USA market. Because of their original patent in the US, they were the only player in the US market for a while. This explains their penetration in the TMS market. Please note that Neuronetics didn’t invent TMS. TMS was developed in Europe, not in the US.
NeuroStar Advanced Therapy uses a figure-of- 8 coil. The NeuroStar system can do standard, 37.5 minutes per session; DASH, 19 minutes per session and TouchStar™ Theta Burst, which is simply iTBS protocol. NeuroStar came late to adopt Theta Burst Stimulation technology. NeuroStar resisted iTBS for a while before finally accepting reality. NeuroStar doesn’t have a navigation system to ensure the placement of the coil. Instead, NeuroStar has a "Contact Sensing" that will alert the treater if contact was lost. They have other fancy trademarks for gimmicks like D-Tect™ which helps the operator "detect" thumb/hand movement during MT determination. Neuronetics has a pay-per-use business model, meaning the TMS clinic has to pay a fee for every TMS session. This, in my opinion, is a poor business model that doesn't serve physicians and patients.
In addition depression treatment for adults, NeuroStar is FDA cleared for treating OCD and
anxious depression. Also, most recently it got FDA-cleared for depression in adolescents. NeuroStar aligned their name with an advocacy that resulted in an inappropriate Medicare policy allowing nurse practitioners to manage TRD with TMS. A subspeciality outside the scope of practice for midlevel providers.
Pros:
- Good market penetration.
- Good support and training.
Cons:
- The pay-per-use business model makes them a “silent partner” with the TMS practice.
- The website directory favors some clinics over others.
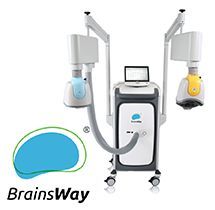
BrainsWay
BrainsWay is based in Israel. BrainsWay TMS devices use Hesel coils. An H coil stimulates deeper in the brain but that comes at the expense of focality. This had been described as “Deep TMS”. The terminology dTMS is not restricted to Hesel coils. It also describes dTMS induced by double cone coils and Halo-circular assembly (HCA) coils. BrainsWay has a patent or a trademark on the terminology deep TMS, which is a descriptive terminology. Any coil can be large enough and powered enough to stimulate deeply. A better description of their product is: non-focal TMS or diffuse-TMS. For this reason, I am calling their H coil dTMS diffuse-TMS.
The main proposal for dTMS is stimulation larger area of the brain cortex. This could increase the chances of “hitting the correct target.” But also, It means delivering a diffuse magnetic field. Often this results in more discomfort during the treatment and, possibly (for the H1 coil), a higher risk of seizure compared to the focal
TMS
treatment with the figure-of-8 coil. BrainsWay is also FDA cleared for iTBS. H coils are incompatible with any navigation systems. For an obvious reason, there is no point in using navigation for targeted treatment when the coil is stimulating broadly.
BrainsWay H1 coil is FDA-cleared for Treatment Resistant Depression and for anxiety associated with depression. BrainsWay H7-coil FDA-cleared for
OCD and MDD. BrainsWay H4 coil is FDA-cleared for smoking cessation. It is interesting how close the H7 and H4 coils are in design and function. Their FDA-clearance paperwork presented the H4 coil as substantially equivalent to the H7 coil. The H4 coil was a 510(k) clearance, not a de novo clearance. Feel free to read the FDA document
here and make your own conclusions. In September 2025, Brainsway announced FDA clearance for accelerated TMS.
Pros:
- Good support and training.
- Indications for MDD + Anxiety, OCD, and Smoking Cessation.
Cons:
- Expensive.
- Superiority complex.
- It might be less tolerable for some patients.
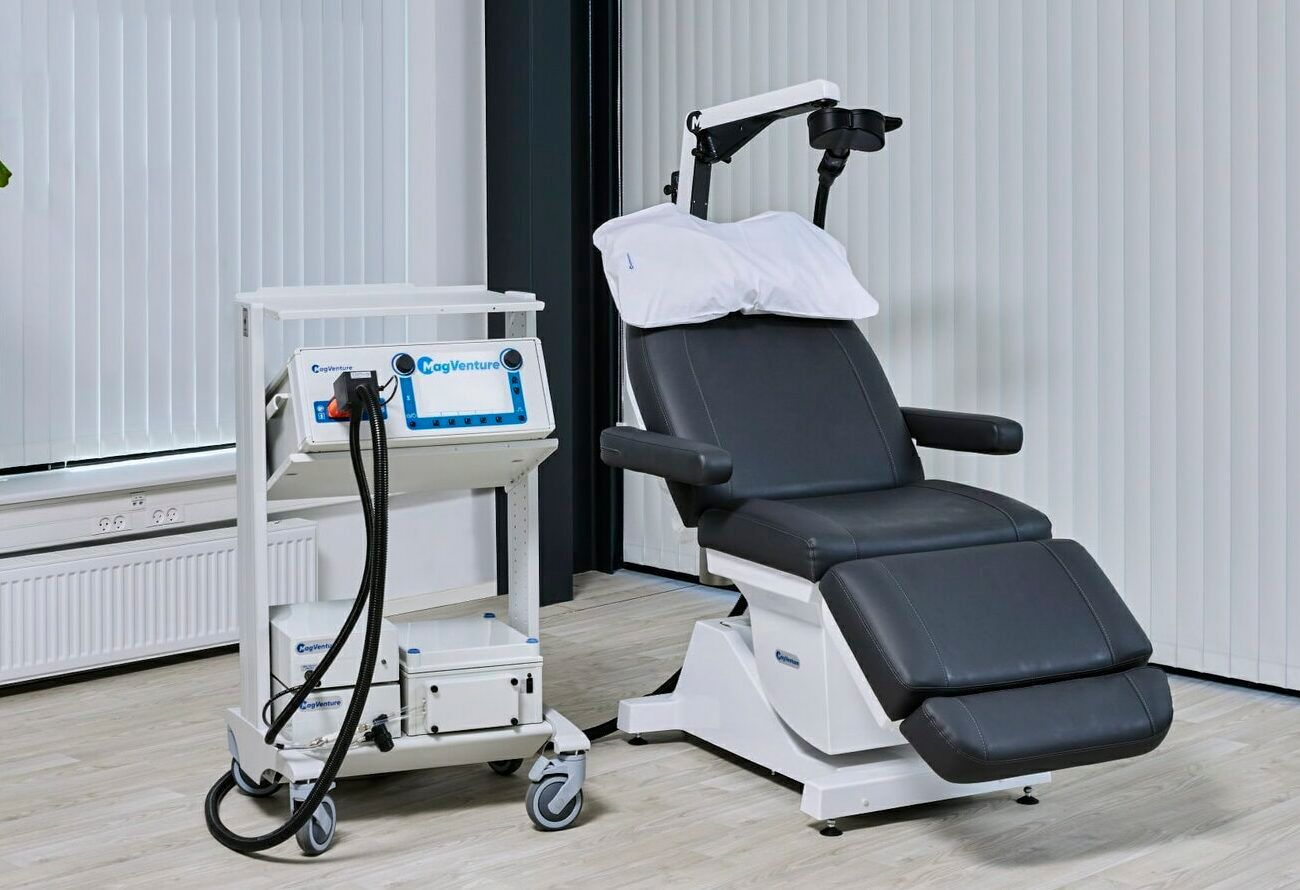
MagVenture
MagVenture is one of the two major pioneers of TMS in Europe (the other one being MagStim). They are based in Denmark. They are proud of the modularity of their system. They have many coils that serve the purpose of research. MagVita is FDA-cleared for rTMS and iTBS. They are the first ones to get the iTBS clearance in 2018. They offer a couple of neuronavigation systems: Atlas™ and Localite. Also, you can add your own neuronavigation system from Brainsight, Vizor2 or Soterix. MagVenture also collaborated with Axilum for a robotic arm. The most recent collaboration was with Magnus, but more on that later.
In 2020, the MagVenture Cool D-B80 coil received a 510(k) FDA clearance for adjunctive treatment of OCD. MagVenture Cool D-B80 coil is the coil equivalent of H7 from Brainsway. But then Neurostar said their F8 coil is similar to D-B80 and got FDA cleared for OCD, then MagVenture said their F8 coil is similar to the Neurostar F8 coil, and MagVenture got another FDA clearance for their F8 coil for OCD. Yes, you read that right, this is how ridiculous the FDA clearance game is. A copy of a copy of a copy going back in full circle, while science and technology can RIP.
MagVenture uses a liquid cooling system. This makes it fast to cool the coil, treating patients back-to-back throughout the day. It is also quieter than air-cooled systems. Liquid cooling and electrical components are not exactly the best combo. You have to be vigilant with maintenance.
Pros:
- Reliable, versatile.
- Good support.
Cons:
- Aesthetically behind, cart-like design.
- Warranties can be expensive upsells.
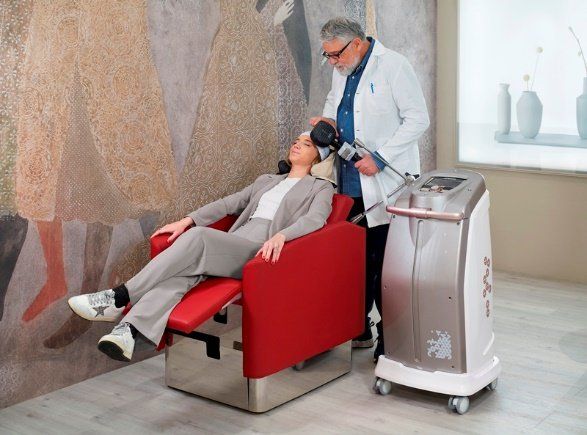
Apollo
Apollo TMS is a product of Mag & More. They are based in Germany. They make fashionable designs for the TMS machine and the TMS chair. They make a unique coil stabilization unit called HANS. The HANS (Head-And-Neck-Support) positioning system ensures that you are reproducibly stimulating the correct treatment spot by following head movement. HANS is good on paper, but not very practical in real life. That being said, they offer alternatives to how you can mount the coil. Apollo is FDA-cleared for both rTMS and iTBS. It is also cleared for OCD.
Apollo uses a unique coating cooling system to cool the coil between sessions. This makes the coil very quiet. This cooling method is not very practical for having multiple back-to-back sessions. Recently, they added an air cooling add-on function to their coil, which seems to cool it faster. Neurocare took over the distribution and support of the Apollo system. They improved support, fixed some apparent flaws, and added air cooling and a new, robust chair. These improvements came with an expense passed on to the consumer by increasing the system's list price and warranty. Apollo has the shortest pulse width of any TMS device. The narrower the pulse width, the more potentiating it can be from the neurobiology standpoint. That finding is not proven to be clinically relevant except in the patient's sense of pain or discomfort during treatment.
Pros:
- Aesthetically pleasing modern design.
- Quiet.
Cons:
- Afterthought cooling system.
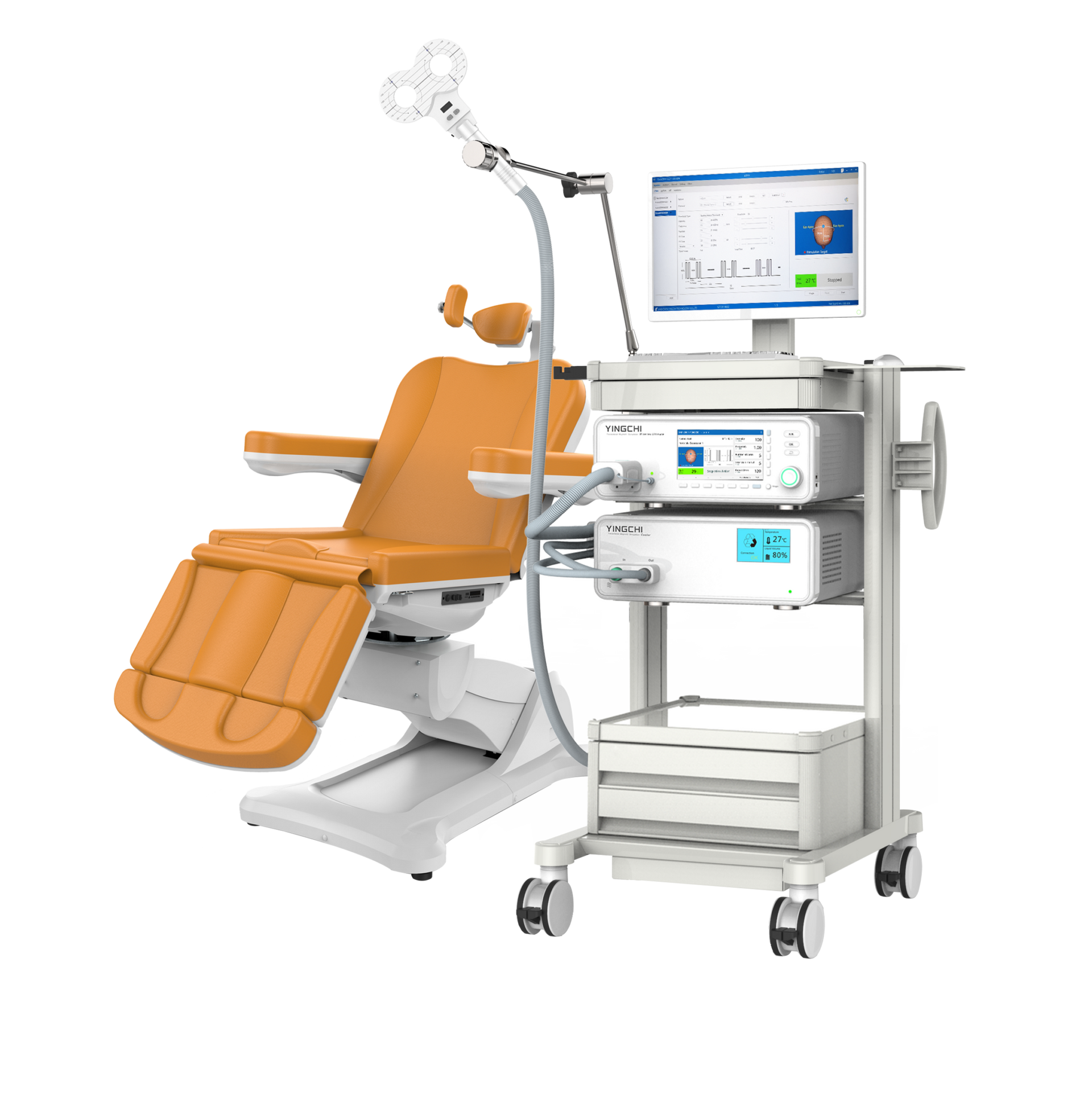
Ultimate rTMS
Ultimate rTMS is a product of Brain Ultimate. The device is manufactured in China by Yingchi TMS. Ultimate rTMS has FDA clearance for coils for MDD and OCD. For MDD, it uses an F8 coil that is liquid-cooled (water-cooled). Their clearance also has an air-cooled coil. So, they basically have two different F8 coils. Ultimate TMS is FDA-cleared for both rTMS and iTBS. The OCD coil is a legit double cone coil. The system is affordable and modular enough to be practical.
Pros:
- Modular design.
- Good value.
Cons:
- Not the best aesthetic!
Blossom
Blossom TMS is a product of Sebers Medical. They are in the US as a branch of a parent company in Germany. The device is manufactured in South Korea. It looks aesthetically pleasing. It uses an F8 coil that is oil cooled, which means effective cooling and relatively quiet stimulation. The coil is permanently attached to the stimulator structure. This means less risk of leaks and potential low maintenance. On the other hand, it means you can’t add different coils for other indications. Blossom can do both rTMS and iTBS. But currently, Blossom is FDA cleared for 10 Hz rTMS only. I understand that they are working on another upgraded TMS system with more indications.
The chair is very promising. It has a massage function and a unique, comfortable “head nest” to stabilize the patient's head during TMS treatment. The unit is very portable—it plugs into a 110-volt outlet. The system is affordable and offers the best value for the money I have seen so far.
Pros:
- Beautiful design.
- Great value.
- Quit.
Cons:
- Only 10 Hz protocol has FDA clearance for MDD despite the device's capability of iTBS.
- A new company, so reliability and support are to be seen.
NexStim
NexStim is based in Finland. SmartFocus by Nexstim was originally made to help Neurosurgeons with brain mapping before performing brain surgery. They brought their TMS system to the US market for depression therapy. NexStim TMS is capable of rTMS, iTBS, and neuronavigation TMS, which they call NBT Navigated Brain Therapy.
SmartFocus requires a specific structural brain MRI to enable the neuronavigation function. Getting an MRI is not a medical necessity. Therefore, insurance may not pay for the MRI portion of the treatment plan, so the patient will have to pay out of pocket, or the TMS clinic will have to eat the cost of such an MRI. Sadly, they don't have a clear business model for selling their system to doctors. They say it is not a pay-per-use model, but you have to buy the disposable head trackers from them for every TMS session you do. That is technically a pay-per-use model!
Pros:
- Cutting-edge NeuroNavigation technology.
- Well-made system.
Cons:
- Needs an MRI. No option to upload an MNI Average Brain MRI.
- The disposable head tracker fee is a walk-around the pay-per-use model.
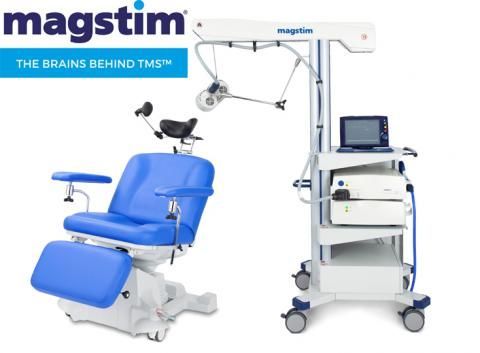
MagStim
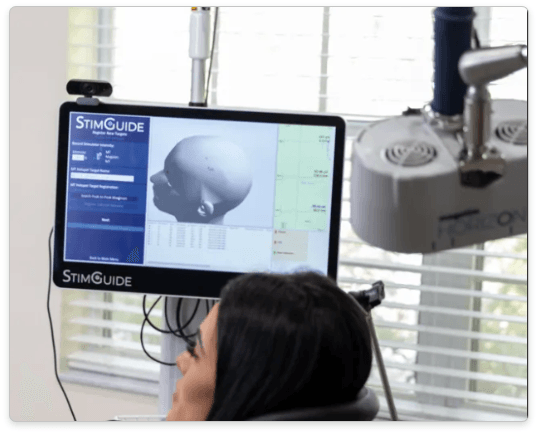
I use MagStim in my practice. MagStim is based in the UK. Dr. Anthony Parker pioneered the first TMS machine in the UK in 1985. In 1990, MagStim's parent company made TMS commercially available. Horizon Performance with Stim-Guide is FDA-cleared for rTMS, iTBS, and navigated TMS. Stim-Guide is their version of neuronavigation. StimGuide doesn’t require an MRI to guide the operator to the treatment location. Non-MRI-guided targeting is less accurate than structural MRI-guided targeting, and structural MRI-guided targeting is less accurate than functional connectivity MRI targeting. Read my detailed article about
navigated TMS if you are interested in learning more about navigated TMS.
MagStim uses an air cooling system. This makes cooling the coil very fast. We never encountered downtime between patients to cool the system. The caveat to air cooling is that it makes the system loud because of the cooling fans.
H3 is their updated TMS system. It builds on the sound foundations of Horizon Performance with StimGuide but adds better user interface software. In addition to rTMS and iTBS FDA clearance for depression, it is also FDA cleared for anxious depression and OCD.
Pros:
- Reliable, versatile, and reasonably priced.
- Excellent technical support and warranty.
- Navigation system.
Cons:
- Unable to upload a patient’s MRI or an MNI Average Brain MRI to improve the navigation accuracy.
- Loud cooling system.
Others:
Magnus Medical got an FDA clearance for the SAINT TMS protocol. In my opinion, apart from the functional connectivity MRI targeting, Accelerated Intermittent Theta Burst Stimulation is the future of TMS. As you are probably aware, SAINT coupled aiTBS with individualized fcMRI targeting the functional DLPFC (the most anticorrelated PFC to the sgACC). Magnus Medical is a new startup that licensed this intellectual property to bring the SAINT protocol to the market. Magnus Medical, as a company, is a huge disappointment. Magnus created a monopoly on accelerated TMS without actually producing the device. The fcMRI component of the SAINT is questionable clinically and might be serving the patent rather than the patient. If Magnus didn't exist, thousands of patients would have received aiTBS at an affordable cost, and dare I say, some lives would have been saved! Anyway, Magnus didn't make a new device; they are using MagVenture hardware and licensing the patent to certain clinics, bloating the cost of aiTBS to unreasonable figures, making it unreachable for most patients.
AMPA is a promising startup that made an almost portable TMS device with a couple of coils: L, which is equivalent to F8 coils, and M, which seems closer to H coils. Interestingly, the L coil is FDA cleared using the iTBS protocol, not the 10 Hz protocol. The company is hyper-focused on the ONE-D protocol, which is the next big thing in accelerated TMS. I like their camera-based navigation system. I am disappointed that they only offer a leasing model to get their device. That might be an issue for docs who want full control of their practices.
ExoMind, which is BTL-995-rTMS, a product of BTL Industries, is another new company that received FDA clearance for a TMS device for depression. They are trying to take a page from the aesthetic clinics and cool sculpting to present TMS as cool lifestyle health care. I don't know how I feel about that. Until I get over that, I will hold myself from reviewing the device.
NeuroQore is a new company that received FDA clearance for a TMS device. I haven't seen the actual device yet.
Cloud TMS is not on my recommended TMS devices list. Please don't contact me asking why I dropped Cloud TMS; contact Cloud TMS and ask them why!
TMS Machines Comparison Table
| MagStim | MagVenture | NexStim | NeuroStar | BrainsWay | Apollo | BlossomTMS | UltimateTMS | |
|---|---|---|---|---|---|---|---|---|
| rTMS | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| iTBS | Yes | Yes | Yes | Yes | Yes | Yes | No* | Yes |
| Navigation | Yes | Yes | Yes | No | No | No | No | No |
| Anxiety | Yes | Yes | No | Yes | Yes | No | No | No |
| OCD | Yes | Yes | No | Yes | Yes | Yes | No | Yes |
| Smoking | No | No | No | No | Yes | No | No | No |
| Cost | $$ | $$ | $$$$ | $$$$ | $$$$ | $$ | $ | $ |
* The device is capable of iTBS but not approved for it.
Above, you can see a table of features compared side by side. Please note that devices compared are the top-tiered FDA cleared system from each manufacturer. This article gets updated annually.